Sessions 2:Chemical Sciences
Peter J. Stang

Character introduction
Positions
Dean, College of Science, University of Utah, 1997-2007
David P. Gardner Chair, 2017 - present
Distinguished Professor of Chemistry, University of Utah, 1992 - present
Chairperson, Department of Chemistry, University of Utah, 1989 - 1995
Assistant, Associate, Full Professor, University of Utah, 1969 -1992
Senior Fellow, Loker Hydrocarbon Research Institute, University of Southern California, 1991-present
Honorary Professor of Chemistry, CAS Institute of Chemistry, Beijing, China, Zheijiang Univ., East China Normal Univ., East China Univ. Of Sc. & Tech., Soochow Univ., Nanjing Univ. of Technology
Major recognitions and honors
2016 Chinese Government "International Cooperation Award in Science and Technology"; 2016 Honorary Doctorate, Texas A&M Univ.; 2015 Chinese Government "Friendship Award"; ACS Priestly Medal 2013; National Medal of Science 2011; F.A. Cotton Medal for Excellence in Chemical Research (2010); Fred Basolo Medal for Outstanding Research in Inorganic Chemistry (2009); ACS Award for Creative Research and Applications of Iodine Chemistry (2007); Foreign Member, Hungarian Academy of Sciences (2007); Linus Pauling Medal (2006); Foreign Member, Chinese Academy of Sciences (2006); Member, National Academy of Sciences (2000); Fellow, American Academy of Arts & Sciences (2002); Titular Member, European Academy of Arts, Sciences & Humanities; ACS George A. Olah Award in Hydrocarbon Chemistry (2003); R. W. Parry Teaching Award in Chemistry (2000); ACS James Flack Norris Award in Physical-Organic Chemistry (1998); Univ. of Utah Rosenblatt Prize for Excellence (1995); ACS Utah Section Award (1994); Governor's Medal for Science and Technology (State of Utah) (1993); D.Sc. honoris causa, Moscow State University, Moscow Russia (1992); D.Sc. honoris causa, Russian Academy of Sciences (1992); Univ. of Utah Chemistry Department "Outstanding Teaching Award" (1989, 1975); Fulbright-Hays Scholar, Zagreb, Yugoslavia (1988);Univ. of Utah Distinguished Research Award (1987); Lady Davis Visiting Professor, Technion, Haifa, Israel (1986, 1997)
Dean, College of Science, University of Utah, 1997-2007
David P. Gardner Chair, 2017 - present
Distinguished Professor of Chemistry, University of Utah, 1992 - present
Chairperson, Department of Chemistry, University of Utah, 1989 - 1995
Assistant, Associate, Full Professor, University of Utah, 1969 -1992
Senior Fellow, Loker Hydrocarbon Research Institute, University of Southern California, 1991-present
Honorary Professor of Chemistry, CAS Institute of Chemistry, Beijing, China, Zheijiang Univ., East China Normal Univ., East China Univ. Of Sc. & Tech., Soochow Univ., Nanjing Univ. of Technology
Major recognitions and honors
2016 Chinese Government "International Cooperation Award in Science and Technology"; 2016 Honorary Doctorate, Texas A&M Univ.; 2015 Chinese Government "Friendship Award"; ACS Priestly Medal 2013; National Medal of Science 2011; F.A. Cotton Medal for Excellence in Chemical Research (2010); Fred Basolo Medal for Outstanding Research in Inorganic Chemistry (2009); ACS Award for Creative Research and Applications of Iodine Chemistry (2007); Foreign Member, Hungarian Academy of Sciences (2007); Linus Pauling Medal (2006); Foreign Member, Chinese Academy of Sciences (2006); Member, National Academy of Sciences (2000); Fellow, American Academy of Arts & Sciences (2002); Titular Member, European Academy of Arts, Sciences & Humanities; ACS George A. Olah Award in Hydrocarbon Chemistry (2003); R. W. Parry Teaching Award in Chemistry (2000); ACS James Flack Norris Award in Physical-Organic Chemistry (1998); Univ. of Utah Rosenblatt Prize for Excellence (1995); ACS Utah Section Award (1994); Governor's Medal for Science and Technology (State of Utah) (1993); D.Sc. honoris causa, Moscow State University, Moscow Russia (1992); D.Sc. honoris causa, Russian Academy of Sciences (1992); Univ. of Utah Chemistry Department "Outstanding Teaching Award" (1989, 1975); Fulbright-Hays Scholar, Zagreb, Yugoslavia (1988);Univ. of Utah Distinguished Research Award (1987); Lady Davis Visiting Professor, Technion, Haifa, Israel (1986, 1997)
Topic: Abiological Self-Assembly: Predesigned Metallacycles and Metallacages via Coordination
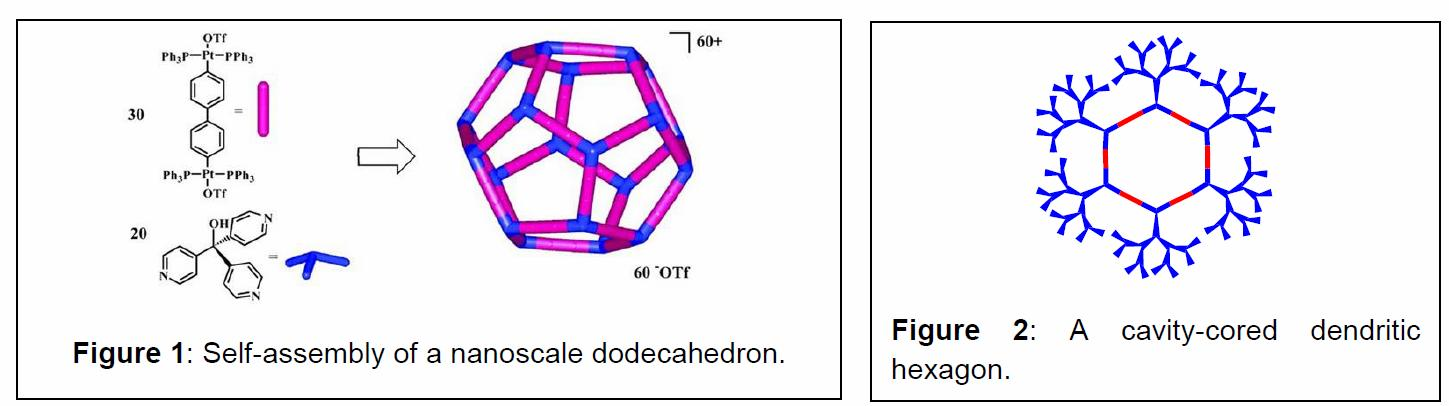
Abstract The use of just two types of building blocks, linear and angular, in conjunction with symmetry considerations allows the rational design of a wide range of metallocyclic polygons and polyhedra via the coordination motif.1-3 We have used this approach to self-assemble a variety of 2D supramolecular polygons such as triangles, rectangles, squares, hexagons, etc. as well as a number of 3D supramolecular polyhedra: truncated tetrahedra, triginal prisms, cubooctahedra4 and dodecahedra.5 An example of the methodology is illustrated in Figure 1. More recently we have functionalized these rigid supramolecular scaffolds with different electroactive, host-guest, dendritic (Figure 2), and hydrophobic/hydrophilic moieties and have investigated the properties of these multifunctionalized supramolecular species.6 Additionally, we have begun to explore the self-assembly of 2D polygons and 3D polyhedra on a variety of surfaces with the aim of developing their potential to be used in device settings.7-12 These novel, supramolecular ensembles are characterized by physical and spectral means. The design strategy, formation, characterization and potential uses of these novel metallocyclic assemblies will be discussed, along with our very recent results.
REFERENCES
1. S.R. Seidel, P. J. Stang, Acc. Chem. Res., 35, 972-983 (2002).
2. S. Leininger, B. Olenyuk, P. J. Stang, Chem. Rev., 100, 853-908 (2000) .
3. P. J. Stang, B. Olenyuk, Acc. Chem. Res., 20, 502-518 (1997).
4. B. Olenyuk, J. A. Whiteford, A. Fechtenkötter, P. J. Stang, Nature, 398, 796-799 (1999).
5. B. Olenyuk, M. D. Levin, J. A. Whiteford, J. E. Shield, P. J. Stang, J. Am. Chem. Soc., 121,
10434-10435 (1999).
6. S. S. Li; B. H. Northrop, Q. H. Yuan, L. J. Wan, P. J. Stang, Acc. Chem. Res., 42, 249-259
(2009).
7. R. Chakrabarty, P. S. Mukherjee, P. J. Stang, Chem. Rev., 111, 6810-6918 (2011).
8. T. R. Cook, Y.-R. Zheng, P.J. Stang, Chem. Rev., 113, 734-777 (2013).
9. T. R. Cook, V. Vajpayee, M. H. Lee, P. J. Stang, K.-W. Chi, Acc. Chem. Res., 46, 2464-2474 (2013).
10. I. V. Grishagin, J. B. Pollock, S. Kushal, T. R. Cook, P. J. Stang, B. Z. Olenyuk, Proc. Nat. Acad. Sci.,
111, 18448-18453 (2014).
11. T. R. Cook, P. J. Stang, Chem. Rev., 115, 7001-7045 (2015).
12. M. L. Saha, X. Yan, P. J. Stang, Acc. Chem. Res., 49, 2527-2539 (2016).

REFERENCES
1. S.R. Seidel, P. J. Stang, Acc. Chem. Res., 35, 972-983 (2002).
2. S. Leininger, B. Olenyuk, P. J. Stang, Chem. Rev., 100, 853-908 (2000) .
3. P. J. Stang, B. Olenyuk, Acc. Chem. Res., 20, 502-518 (1997).
4. B. Olenyuk, J. A. Whiteford, A. Fechtenkötter, P. J. Stang, Nature, 398, 796-799 (1999).
5. B. Olenyuk, M. D. Levin, J. A. Whiteford, J. E. Shield, P. J. Stang, J. Am. Chem. Soc., 121,
10434-10435 (1999).
6. S. S. Li; B. H. Northrop, Q. H. Yuan, L. J. Wan, P. J. Stang, Acc. Chem. Res., 42, 249-259
(2009).
7. R. Chakrabarty, P. S. Mukherjee, P. J. Stang, Chem. Rev., 111, 6810-6918 (2011).
8. T. R. Cook, Y.-R. Zheng, P.J. Stang, Chem. Rev., 113, 734-777 (2013).
9. T. R. Cook, V. Vajpayee, M. H. Lee, P. J. Stang, K.-W. Chi, Acc. Chem. Res., 46, 2464-2474 (2013).
10. I. V. Grishagin, J. B. Pollock, S. Kushal, T. R. Cook, P. J. Stang, B. Z. Olenyuk, Proc. Nat. Acad. Sci.,
111, 18448-18453 (2014).
11. T. R. Cook, P. J. Stang, Chem. Rev., 115, 7001-7045 (2015).
12. M. L. Saha, X. Yan, P. J. Stang, Acc. Chem. Res., 49, 2527-2539 (2016).
Previous Kuiling Ding
Next Benzhong Tang








